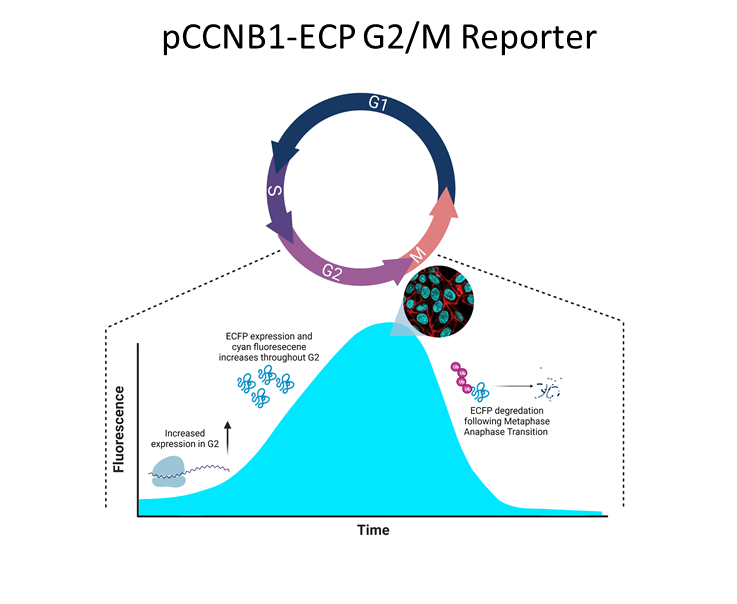
Progression through the G2 phase of the cell cycle has been technically difficult to study due to the lack of physical markers specific to this phase. G2 contains several checkpoints and key protein interactions necessary for the progression of the cell into mitosis. Using modified fluorescent protein systems, our lab has created a cyan fluorescent protein (ECFP) to act as a new G2/M reporter tool to help measure the progression of the cell through G2 without disrupting the cell cycle. The reporter uses a Cyclin B1 promoter which enhances protein expression during G2, mimicking Cyclin B1 expression. As the cell progresses through G2, more ECFP protein is produced leading to increased cyan fluorescence emission within the cell. This acts as a visual marker to indicate the progression of the cell through G2. The protein itself is also fused to a small D-box domain (also found on Cyclin B1) which ensures its degradation at the metaphase–anaphase transition and indicates the cell has progressed into mitosis. Similar reporter systems have been used in the past, but often include the cytoplasmic retention sequence (CRS) domain of Cyclin B1 which is a region known to interact with a number of other intracellular proteins. This can pose problems for researchers and their protein of interest as the CRS domain may lead to unintentional interactions with the reporter and cause unexpected side effects. Our reporter system removes the CRS to help prevent these unwanted interactions. You can read more about this reporter here.
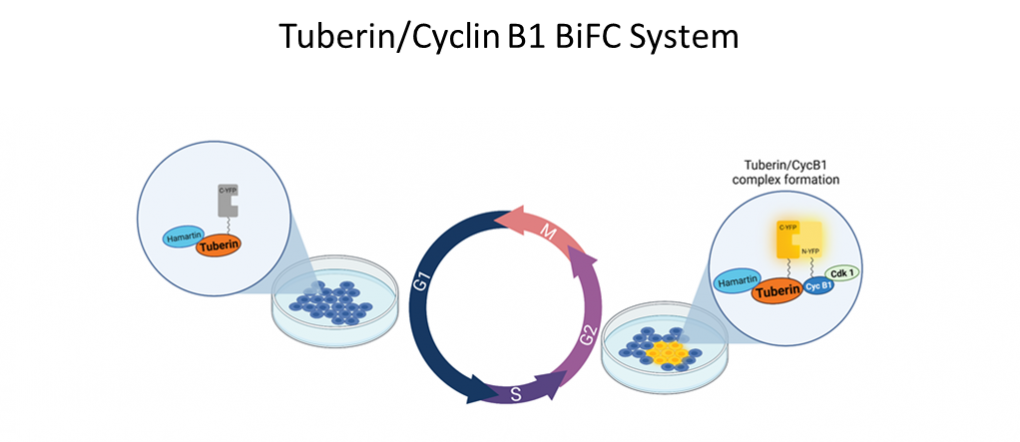
In order to study Tuberin/Cyclin B1 interaction, Porter Lab uses advanced molecular tools such as BiFC. BiFC measures protein interaction through the formation of a BiFC complex which generates fluorescence when proteins of interest interact with one another. The genes of the proteins of interest are modified to be attached to a C-terminal or N-terminal fluorescent protein fragment using a flexible linker. These fragments are a part of a larger fluorescent protein. When the fragments are rejoined, fluorescent light is produced. Our lab has constructed vectors with modified Tuberin and Cyclin B1 genes conjugated to yellow fluorescent protein fragments. After the vector’s transfection into live cells, the interaction of Tuberin and Cyclin B1 at the G2/M checkpoint can be visualized. Tuberin and Cyclin B1’s close association during G2 allows for the reformation of the BiFC complex and yellow fluorescence to be observed.
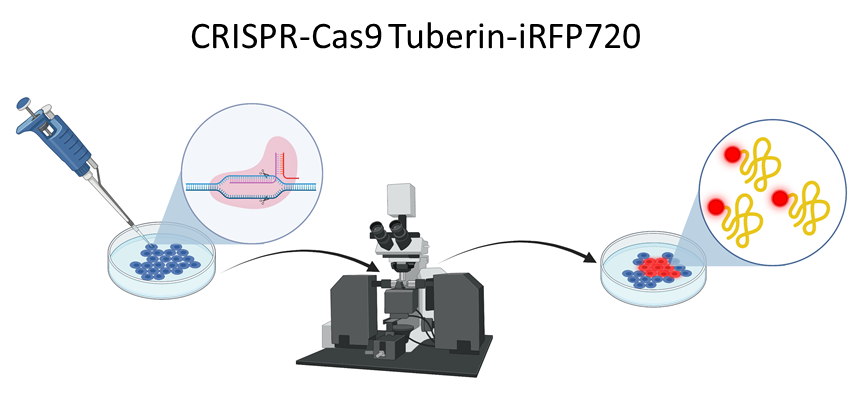
Tuberin has been known to localize both within the cytoplasm and nucleus where it interacts with a variety of different proteins. To better understand Tuberin’s cellular localization and interactions it is essential to develop necessary tools for the imaging of Tuberin within the cell. To visualize Tuberin in the diverse cell compartments, our lab created a new HEK-293 cell line, using the CRISPR-Cas9 technique, where Tuberin is tagged to iRFP720 fluorescent protein. This new cell line can be used in immunofluorescence experiments to detect the localization of Tuberin and also in flow cytometry where cells expressing Tuberin-iRFP720 can be sorted and analyzed.
